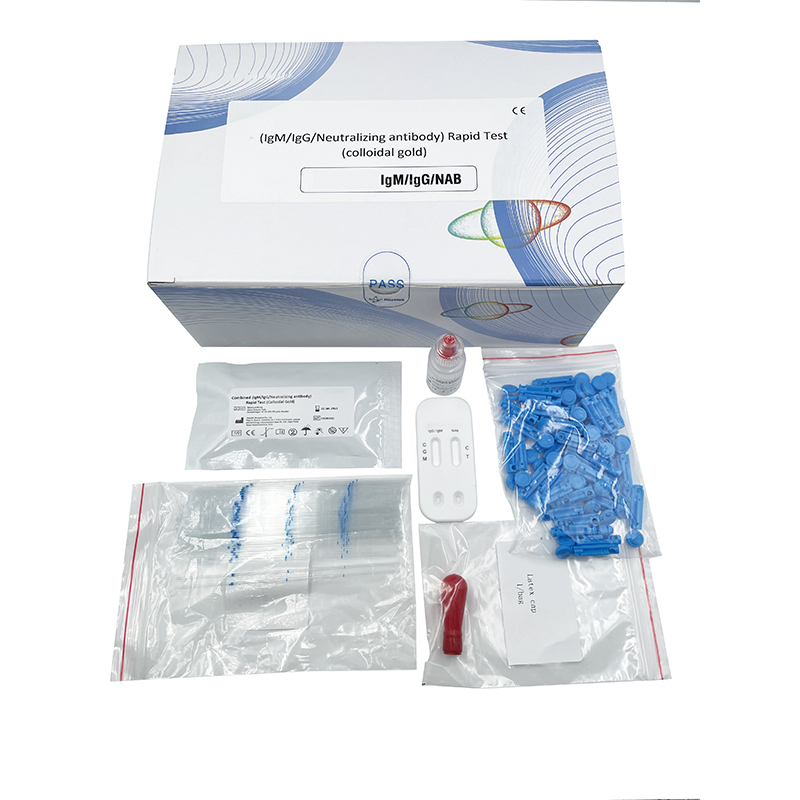
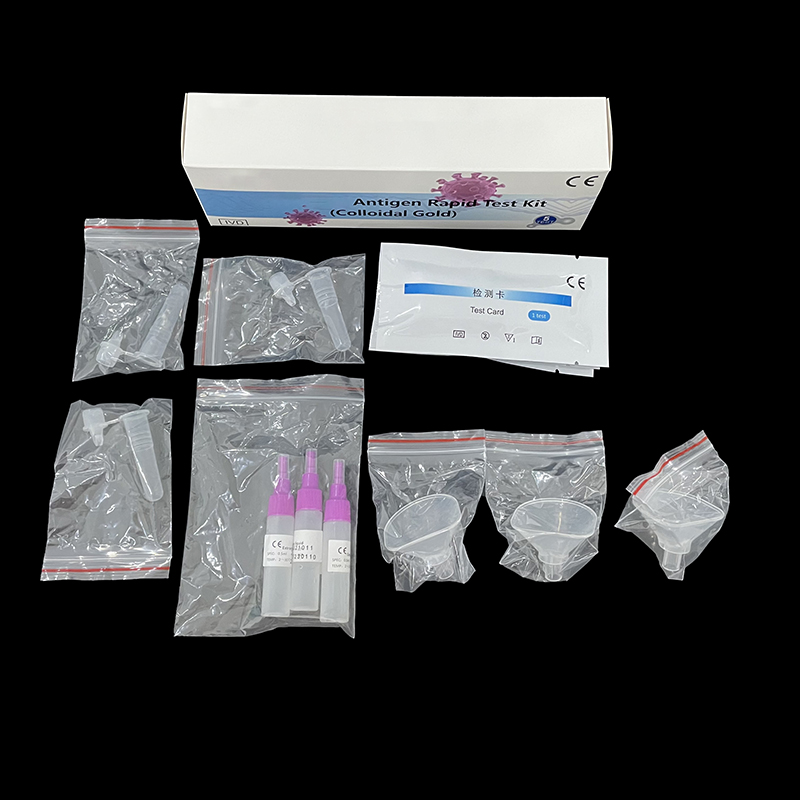
COVID-19 IgM/IgG Antibodies Rapid Test OEM Supplier for Quality Testing
Our COVID-19 IgM/IgG Antibodies Rapid Test offers an efficient solution for those seeking reliable results. As a supplier, I understand how crucial it is for businesses to have access to high-quality testing kits. This rapid test enables quick detection of both IgM and IgG antibodies, helping you make informed decisions swiftly. I assure you that our product is perfect for OEM partnerships, supporting your branding needs without compromising on quality. This assay is designed to provide clear results within minutes, easing the pressure in critical situations. When you choose to source from us, you’re getting a tested and trusted resource that meets rigorous industry standards. Let’s work together to enhance your offerings and ensure the health safety of your clientele. Reach out to me if you’re interested in bulk orders or if you have any specific requirements. Your satisfaction and trust are what drive us to deliver excellence.
COVID-19 IgM/IgG Antibodies Rapid Test Market Leader Where Innovation Meets 2025
The COVID-19 pandemic has reshaped the landscape of healthcare and diagnostics, catalyzing innovations in rapid testing solutions. With the growing demand for reliable and swift testing methods, the COVID-19 IgM/IgG Antibodies Rapid Test market has become a focal point for healthcare providers, governmental organizations, and global procurement buyers. As we aim for a safer future, the ability to quickly identify and manage infections plays a critical role in public health strategies, especially in the face of emerging variants. Innovation in testing technology has led to highly accurate and easy-to-use rapid test kits that enable users to detect antibodies indicative of past or present infection. These advancements not only enhance diagnostic capabilities but also streamline supply chains and reduce dependence on laboratory-based testing, making it particularly advantageous in remote or under-resourced areas. Procurement professionals seeking to equip healthcare systems and institutions with effective tools will find a wide array of options tailored to meet diverse needs, promising both quality and efficiency. As we look towards 2025, the integration of AI and data analytics in test development and deployment will likely transform how rapid tests are utilized in both clinical and non-clinical settings. This evolution presents exciting opportunities for global procurement strategists to invest in innovative products that not only address current challenges but also prepare for future public health needs. Engaging with leading providers in the market can pave the way for enhanced healthcare solutions that prioritize speed, accuracy, and accessibility in combating infectious diseases.
COVID-19 IgM/IgG Antibodies Rapid Test Market Leader Where Innovation Meets 2025
| Region | Market Share (%) | Growth Rate (CAGR %) | Major Trends | Innovation Highlights |
|---|---|---|---|---|
| North America | 40% | 15% | Increased home testing kits, digital integration | AI-driven diagnostics |
| Europe | 35% | 12% | European regulations supporting rapid tests | Mobile health applications |
| Asia-Pacific | 20% | 20% | Emergence of local manufacturers | Novel assay technologies |
| Latin America | 3% | 10% | Growing demand for accessible healthcare | Point-of-care testing advancements |
| Middle East & Africa | 2% | 8% | Investments in healthcare infrastructure | Rapid test kit innovations |
Related Products