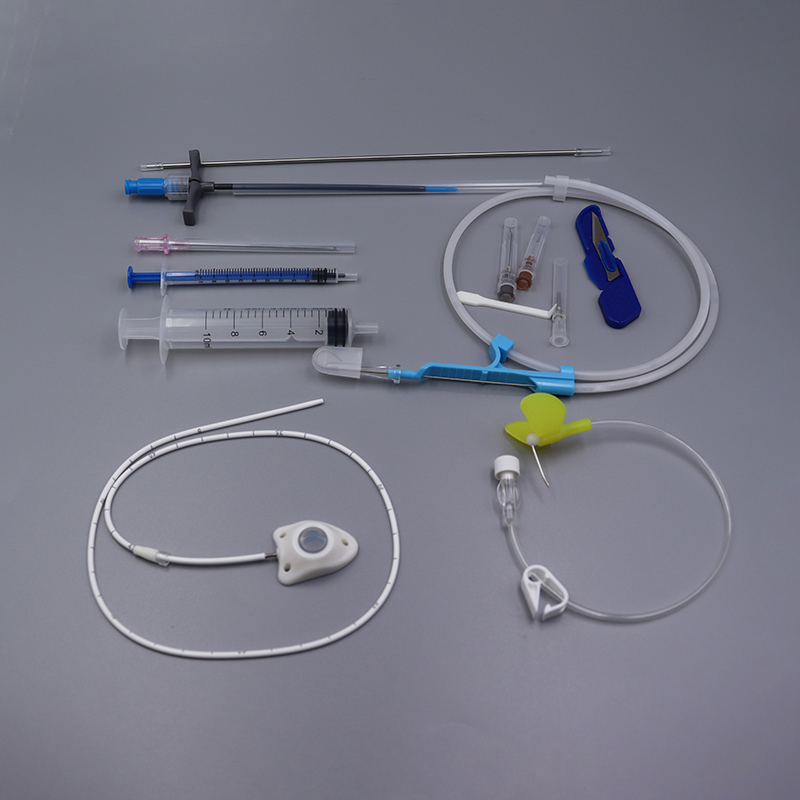
[Application] The vascular device implantable port is suitable for guided chemotherapy for a variety of malignant tumors, prophylactic chemotherapy after tumor resection and other lesions requiring long-term local administration.
[Specification]
| Model | Model | Model |
| I-6.6Fr×30cm | II-6.6Fr×35cm | III- 12.6Fr×30cm |
【Performance】The self-sealing elastomer of the injection holder allows 22GA needles of implantable port for 2000 times puncture. The product is made entirely of medical polymers and is metal-free.Catheter is X-ray detectable. Sterilized by ethylene oxide, single-use. Anti-reflux design.
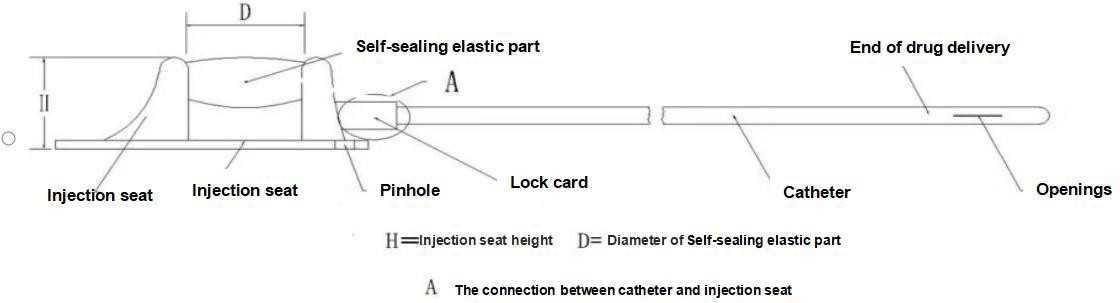
【Structure】This device consists of an injection seat (including self-sealing elastic parts, puncture restriction parts, locking clips) and a catheter, and the Type II product is equipped with a locking clip booster The catheter and self- sealing elastic membrane of the implantable drug delivery device are made of medical silicone rubber, and the other components are made of medical polysulfone. The following diagram introduces the main structure and component names of the product, regard type I as an example.
【Contraindications】
1) Psychological or physical unsuitability for surgery in general conditions
2) Severe bleeding and coagulation disorders.
3) White blood cell count less than 3×109/L
4) Allergic to contrast media
5) Combined with severe chronic obstructive pulmonary disease.
6) Patients with known or suspected allergy to the materials in the device package.。
7) Presence or suspicion of device-related infection, bacteraemia or sepsis.
8) Radiotherapy at the site of intended insertion.
9) Imaging or injection of embolic drugs.
【Manufacturedate】 See product label
【Expirydate】 See product label
【Method of application】
- Prepare the implantable port device and check if the expiration date is exceeded; remove the inner package and check if the package is damage.
- Should use aseptic techniques to cut open the inner package and remove the product for prepare to use.
- The use of implantable port devices is described separately for each model as follows.
TypeⅠ
- Flushing, venting, leak testing
Use a syringe (needle for implantable port device) to puncture the implantable port device and inject 5mL-10mL of physiological saline to flush the injection seat and catheter lumen and exclude. If no or slow liquid is found, twist the drug delivery end of the catheter (distal end) by hand to open the drug delivery port; then Fold closed the drug delivery end of the catheter, continue to push saline (pressure not more than 200kPa), observe whether there is leakage from the injection seat and catheter connection, after all normal After everything is normal, the catheter can be used.
- Cannulation and ligation
According to the intraoperative investigation, insert the catheter (drug delivery end) into the corresponding blood supply vessel according to the location of the tumor, and use non-absorbable sutures to properly ligate the catheter to the vessel. The catheter should be properly ligated (two or more passes) and fixed.
- chemotherapy and sealing
Intraoperative chemotherapy drug can be injected once according to the treatment plan; it is recommended that the injection seat and catheter lumen be flushed with 6-8 mL of physiological saline, followed by 3 mL~5 mL The catheter is then sealed with 3mL to 5mL of heparin saline at 100U/mL to 200U/mL.
- Injection seat fixation
A subcutaneous cystic cavity is created at a place of support, which is 0.5 cm to 1 cm from the skin surface, and the injection seat is placed into the cavity and fixed, and the skin is sutured after strict hemostasis. If the catheter is too long, it can be coiled into a circle at the proximal end and fixed properly.
TypeⅡ
1.Flushing and venting
Use a syringe (needle for implantable port device) to inject saline into the injection seat and catheter respectively to flush and remove the air in the lumen, and observe whether the conduction fluid is smooth.
2. Cannulation and ligation
According to the intraoperative investigation, insert the catheter (drug delivery end) into the corresponding blood supply vessel according to the location of the tumor, and properly ligate the catheter with the vessel with non- absorbable sutures. The catheter should be properly ligated (two or more passes) and fixed.
3. Connection
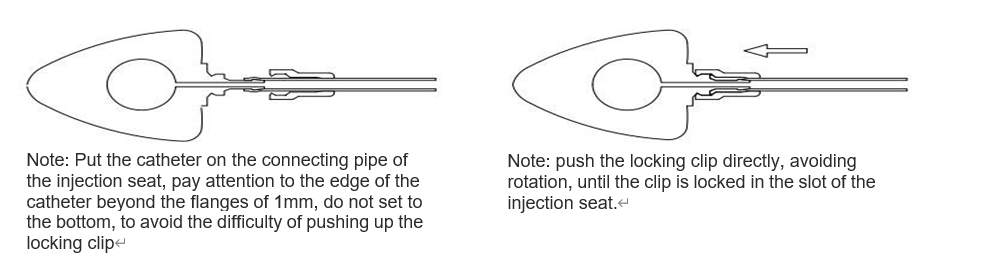
Determine the required catheter length according to the patient’s condition, cut off the excess from the proximal end of the catheter (non-dosing end), and insert the catheter into the injection seat connection tube using
Use the locking clip booster to push the locking clip firmly into tight contact with the injection holder. Then gently pull the catheter outward to check that it is secure. This is done as shown in
Figure below.
4. Leak test
4. After the connection is completed, fold and close the catheter at the back of the locking clip and continue to inject saline into the injection seat with a syringe (needle for implantable drug delivery device) (pressure over 200kPa). (pressure not more than 200kPa), observe whether there is leakage from the injection block and catheter
connection, and use only after everything is normal.
5. Chemotherapy, sealing tube
Intraoperative chemotherapy drug can be injected once according to the treatment plan; it is recommended to flush the injection base and catheter lumen with 6~8mL of physiological saline again, and then use 3mL~5mL of physiological saline.
The catheter is then sealed with 3mL to 5mL of heparin saline at 100U/mL to 200U/mL.
6. Injection seat fixation
A subcutaneous cystic cavity was created at a place of support, 0.5 cm to 1 cm from the skin surface, and the injection seat was placed into the cavity and fixed, and the skin was sutured after strict hemostasis.
Type Ⅲ
A syringe (special needle for implantable port device) was used to inject 10mL ~ 20mL normal saline into the implantable drug delivery device to flush the injection seat and the cavity of the catheter, and remove the air in the cavity, and observe whether the fluid was unobtrusive.
2. Cannulation and ligation
According to the intraoperative exploration, insert the catheter along the abdominal wall, and the open part of the drug delivery end of the catheter should enter the abdominal cavity and be as close to the tumor target as possible. Choose 2-3 points to ligate and fix the catheter.
3. chemotherapy, sealing tube
Intraoperative chemotherapy drug can be injected once according to the treatment plan, and then the tube is sealed with 3mL~5mL of 100U/mL~200U/mL heparin saline.
4. Injection seat fixation
A subcutaneous cystic cavity was created at a place of support, 0.5 cm to 1 cm from the skin surface, and the injection seat was placed into the cavity and fixed, and the skin was sutured after strict hemostasis.
Drug infusion and care
A. Strictly aseptic operation, correct selection of the injection seat location before injection, and strict disinfection of the injection site. B. When injecting, use a needle for implantable port device, a syringe of 10 mL or more, with the left hand index finger touching the puncture site and the thumb tensing the skin while fixing the injection seat, with the right hand holding the syringe vertically into the needle, avoiding shaking or rotating, and slowly injecting saline 5 mL~10 mL when there is a sense of falling and the tip of the needle subsequently touches the bottom of the injection seat, and check whether the drug delivery system is smooth (if it is not smooth, you should first check whether the needle is blocked). Observe whether there is any elevation of the surrounding skin when pushing.
C. Push the chemotherapeutic drug slowly after confirming that there is no error. During the pushing process, pay attention to observe whether the surrounding skin is elevated or pale, and whether there is local pain. After the drug is pushed, it should be kept for 15s~30s.
D. After each injection, it is recommended to flush the injection seat and catheter lumen with 6~8mL of physiological saline, and then seal the catheter with 3mL~5mL of 100U/mL~200U/mL of heparin saline, and when the last 0.5mL of heparin saline is injected, the drug should be pushed while retreating, so that the drug introduction system is filled with heparin saline to prevent drug crystallization and blood coagulation in the catheter. The catheter should be flushed with heparin saline once every 2 weeks during the interval of chemotherapy.
E. After the injection, disinfect the needle eye with medical disinfectant, cover it with sterile dressing, and pay attention to keep the local area clean and dry to prevent infection at the puncture site.
F. Pay attention to the patient’s reaction after drug administration and observe closely during drug injection.
【Caution, warning and suggestive content】
- This product is sterilized by ethylene oxide and is valid for three years.
- Please read the instruction manual before use to ensure the safety of use.
- The use of this product must comply with the requirements of the relevant codes of practice and regulations of the medical sector, and the insertion, operation and removal of these devices should be restricted to certified physicians.The insertion, operation and removal of these devices are restricted to certified physicians, and post-tube care should be performed by qualified medical personnel.
- The entire procedure must be performed under aseptic conditions.
- Check the expiration date of the product and the inner packaging for damage before the procedure.
- After use, the product may cause biological hazards. Please follow accepted medical practice and all relevant laws and regulations for handling and treatment.
- Do not use excessive force during intubation and insert the artery accurately and quickly to avoid vasospasm. If intubation is difficult, use your fingers to turn the catheter from side to side while inserting the tube.
- The length of the catheter placed in the body should be appropriate, too long is easy to curl into an angle, resulting in poor ventilation, too short is when the patient violent activities have the possibility of dislodging from the vessel. If the catheter is too short, it may dislodge from the vessel when the patient moves vigorously.
- The catheter should be inserted into the vessel with more than two ligatures and appropriate tightness to ensure smooth drug injection and to prevent the catheter from slipping off.
- If the implantable port device is type II, the connection between the catheter and the injection seat must be firm. If intraoperative drug injection is not required, normal saline test injection should be used for confirmation before suturing the skin.
- When separating the subcutaneous area, close hemostasis should be performed to avoid the formation of local hematoma, fluid accumulation or secondary infection after surgery; the vesicular suture should avoid the injection seat.
- α-cyanoacrylate medical adhesives can cause damage to the injection base material; do not use α- cyanoacrylate medical adhesives when treating the surgical incision around the injection base. Do not use α-cyanoacrylate medical adhesives when dealing with surgical incisions around the injection base.
- Use extreme caution to avoid leakage of the catheter due to accidental injury from surgical instruments.
- When puncturing, the needle should be inserted vertically, a syringe with a capacity of 10mL or more should be used, the drug should be injected slowly, and the needle should be withdrawn after a short pause. The pushing pressure should not exceed 200kPa.
- Only use special needles for implantable drug delivery devices.
- When a longer infusion or drug replacement is required, it is appropriate to use a single-use implantable drug delivery device with hose special infusion needle or tee, in order to reduce the number of punctures and reduce the impact on the patient.
- Reduce the number of punctures, reduce the damage to the patient’s muscle and self-sealing elastic parts. During the period of discontinuation of drug injection, anticoagulant injection is required once every two weeks.
- This product is a single-use, sterile, non-pyrogenic product, destroyed after use, reuse is strictly prohibited.
- If the inner package is damaged or the product expiration date has been exceeded, please return it to the manufacturer for disposal.
- The number of punctures for each injection block should not exceed 2000 (22Ga). 21.
- The minimum flushing volume is 6ml
【Storage】
This product should be stored in a non-toxic, non-corrosive gas, well-ventilated, clean environment and prevented from extrusion.
Post time: Mar-25-2024